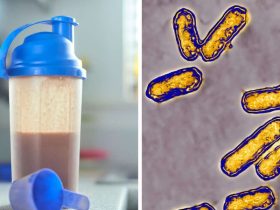
According to the Food and Drug Administration, a “black mold-like substance” was identified at a Tom’s of Maine production facility, where the brand’s toothpaste was made using bacterially contaminated water.
The FDA claimed in a letter to Colgate-Palmolive, the company that owns the brand, that water samples used to create Tom’s Simply White Clean Mint Paste included a form of bacteria known as Pseudomonas aeruginosa, which can cause lung and blood infections.
The FDA states that the water samples were collected from June 2021 to October 2022.
A batch of Tom’s Wicked Cool! Anticavity Toothpaste also contained Paracoccus yeei, another strain of bacteria.
“Water is a major ingredient in many of your OTC drug products. It is essential that you employ a water system that is robustly designed, and that you effectively control, maintain, and monitor the system to ensure it consistently produces water suitable for pharmaceutical use,” the FDA stated in the Nov. 5 letter.
According to the FDA’s letter, the examination of the Sanford, Maine business revealed “a black mold-like substance,” in many places close to manufacturing equipment.
According to the FDA, the inspection took place in May of this year.
Additionally, a batch of Tom’s Silly Strawberry Anticavity toothpaste, which the firm claims is intended for “kids and parents,” was discovered to have a “powder residue” close by.
“It is essential that your facility is in a good state of repair and sanitary conditions are maintained to protect drug products from potential routes of contamination,” the FDA stated.
Colgate-Palmolive was asked by the agency to submit the findings of the testing and provide more documentation of manufacturing processes that included a “thorough review of all microbiological hazards”.
A Colgate-Palmolive representative told that the business is collaborating with the FDA to address the concerns brought up during the inspection.
Read Also: California Woman Receives $1M Settlement After Police Dog Bites Her Scalp
“We have always tested finished goods before they leave our control, and we remain fully confident in the safety and quality of the toothpaste we make,” the company stated.
“In addition, we have engaged water specialists to evaluate our systems at Sanford, have implemented additional safeguards to ensure compliance with FDA standards, and our water testing shows no issues,” the company continued.










Leave a Reply