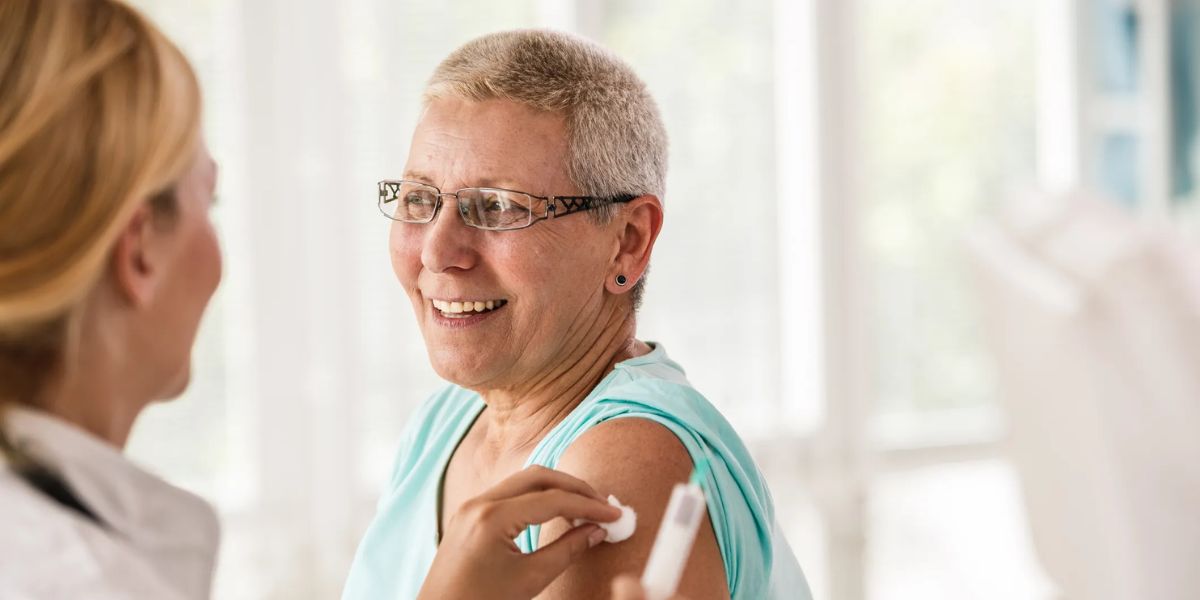
On Wednesday, U.S. health officials advised anyone aged 50 and above to receive a vaccination against bacteria that can cause pneumonia and other serious infections.
A scientific advisory council issued the recommendation, which the Centers for Disease Control and Prevention subsequently adopted. The minimum suggested age for older persons to receive the vaccination was lowered by the ruling, from 65.
“Now is a great time to get vaccinated against pneumococcal disease in preparation for the winter respiratory season,” CDC Director Dr. Mandy Cohen stated.
Earlier in the day, the advisory committee met in Atlanta and decided 14-1 to make the modification. It encourages health insurance to cover recommended vaccinations and is commonly followed by physicians.
The government’s recommendations for pneumococcal vaccinations are sometimes referred to as the most complex. As long as they have never received a pneumococcal disease vaccination, the CDC presently advises vaccinations for adults 65 years of age or older and children under the age of five.
Additionally, officials advise the vaccinations for adults and children who are more susceptible to pneumococcal disease, such as those with diabetes, chronic liver disease, or compromised immune systems.
Pneumococci bacteria, which can cause severe infections in the lungs and other regions of the body, come in over 100 different varieties. About 30,000 cases of invasive pneumococcal disease, which includes blood infections, inflammation of the brain and spine, and other disorders, occur in the United States each year. People aged 50 to 64 account for about 30% of instances.
Pharmaceutical companies have been developing newer pneumococcal vaccines that target a dozen or more varieties in a single shot since the first one was licensed in the United States in 1977.
The popularity of many vaccinations has fluctuated, such as Pfizer’s Prevnar 13, which was formerly a best-seller but is now unavailable.
Currently, four vaccinations are being used. This year, Merck’s Capvaxive, which can cost around $300 per dose and protects against 21 types of pneumococcal infections, including eight that are not present in other vaccines, was approved by the U.S. Food and Drug Administration.
According to a Merck representative, it was created especially to help guard against the kinds of germs that cause most serious illnesses in people 50 years of age and older.
In June, the CDC advisory group said that individuals who were at a higher risk should consider the vaccine. The committee also discussed the potential of reducing the recommended age for older persons at that time.
Read Also: We’re Done’: Florida Residents Set to Leave State, Sell Homes After Hurricane Season
“Pneumococcal has been a very confusing recommendation for many, many years and it’s hard to have a new recommendation every two or three years,” stated Dr. Jamie Loehr, who chairs the committee’s pneumococcal working group. The only one to vote against the plan was him.









Leave a Reply